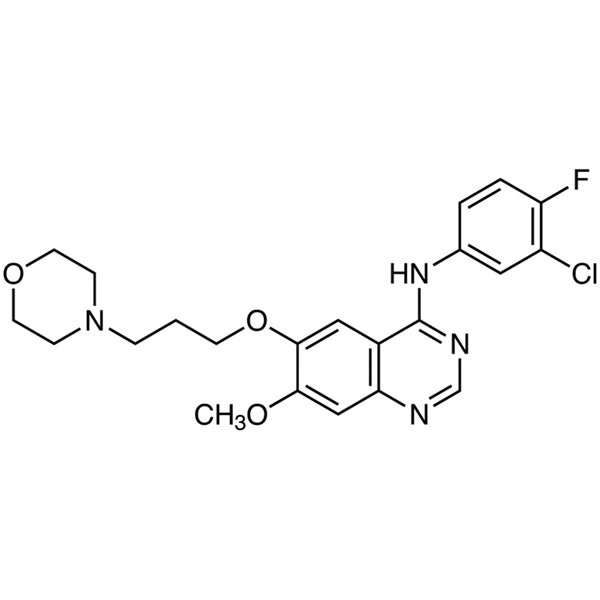
Gefitinib CAS 184475-35-2 Purity >99.5% (HPLC)
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Gefitinib (CAS: 184475-35-2) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Gefitinib and intermediates, Please contact: alvin@ruifuchem.com
| Chemical Name | Gefitinib |
| Synonyms | Gefitinib Free Base; Iressa; ZD1839; ZD-1839; N-(3-Chloro-4-Fluorophenyl)-7-Methoxy-6-(3-Morpholinopropoxy)quinazolin-4-Amine; N-(3-Chloro-4-Fluorophenyl)-7-Methoxy-6-[3-(4-Morpholinyl)propoxy]-4-Quinazolinamine |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 184475-35-2 |
| Molecular Formula | C22H24ClFN4O3 |
| Molecular Weight | 446.91 g/mol |
| Melting Point | 194.0 to 198.0℃ |
| Density | 1.322±0.06 g/cm3 |
| Solubility | Insoluble in Water. Soluble in DMSO |
| Storage Temp. | Room Temperature |
| Shipping | Ambient |
| COA & MSDS | Available |
| Origin | Shanghai, China |
| Product Categories | API (Active Pharmaceutical Ingredient) |
| Brand | Ruifu Chemical |
| Items | Inspection Standards | Results |
| Appearance | White to Off-White Powder | Complies |
| Loss on Drying | <0.50% | 0.13% |
| Residue on Ignition | <0.20% | 0.06% |
| Single Impurity | <0.10% | 0.09% |
| Total Impurities | <0.50% | 0.20% |
| Heavy Metals (Pb) | ≤10ppm | <10ppm |
| Gefitinib Purity | >99.5% (HPLC) | 99.80% |
| Infrared Spectrum | Consistent with Structure | Complies |
| 1H NMR Spectrum | Consistent with Structure | Complies |
| Conclusion | The product has been tested and complies with the given specifications | |
Package: Fluorinated Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry and well-ventilated warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only.
None of the products will be supplied to countries in which this could be in conflict with the existing patents. However the final responsibility lies with the Buyer.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
| Safety Description | 24/25 - Avoid contact with skin and eyes. |
| HS Code | 2934999099 |
Gefitinib (CAS: 184475-35-2) is a highly specific anti-tumor targeted therapeutic drug developed by AstraZeneca, UK. It is the first molecular targeted drug for the treatment of non-small cell lung cancer. It works by selectively inhibiting the signal transduction pathway of epidermal growth factor receptor tyrosine kinase (EGFR-TK). Epidermal growth factor (EGF) is a polypeptide with a relative molecular mass of 6.45 × 103, which can combine with epidermal growth factor receptor (EGFR) on the target cell membrane to produce biological effects. EGFR is a tyrosine kinase (TK) type receptor. When it binds to EGF, it can promote TK activation in the recipient body, resulting in autophosphorylation of receptor tyrosine residues, providing continuous division signals to cells, causing cell proliferation and differentiation. EGFR is abundant in human tissues and is highly expressed in malignant tumors. By blocking the EGFR signaling pathway on the cell surface, gefitinib hinders tumor growth, metastasis and angiogenesis, and can induce apoptosis of tumor cells. In August 2002, gefitinib was first marketed in Japan as a first-line treatment for non-small cell lung cancer under the trade name Iressa. In May 2003, the U.S. Food and Drug Administration approved gefitinib as the third-line monotherapy for patients with advanced non-small cell lung cancer who were ineffective with platinum-based anticancer drugs and docetaxel chemotherapy. At present, it has been approved by Australia, Japan, Argentina, Singapore and South Korea for the treatment of advanced non-small cell lung cancer. On February 28, 2005, the China Food and Drug Administration approved gefitinib for the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) that had previously received chemotherapy. It is not currently approved for use as first-line therapy for advanced NSCLC. On July 1, 2009, the European Medicines Agency officially approved gefitinib for the first-line, second-line and third-line treatment of locally advanced or metastatic non-small cell lung cancer with EGFR gene mutations in adults.
-
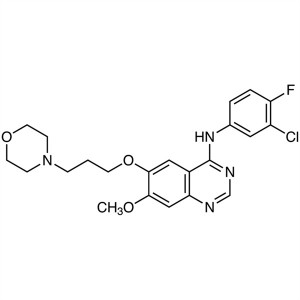
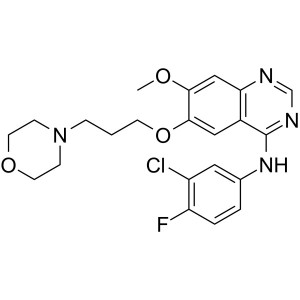
Gefitinib CAS 184475-35-2 Purity >99.5% (HPLC)
-
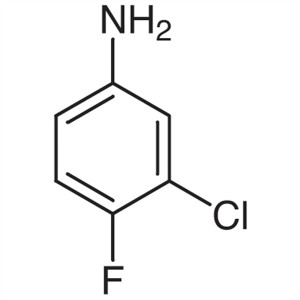
3-Chloro-4-Fluoroaniline CAS 367-21-5 Gefitinib...
-
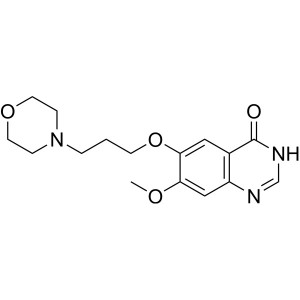
Gefitinib Intermediate CAS 199327-61-2 Purity >...
-
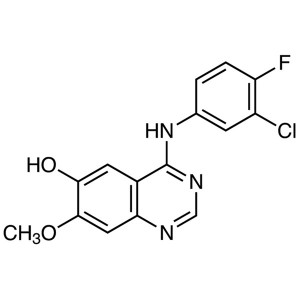
Gefitinib Intermediate CAS 184475-71-6 Purity >...
-
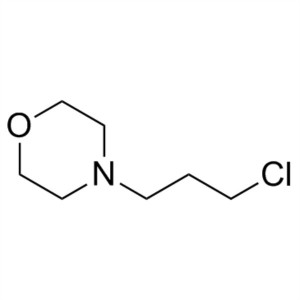
4-(3-Chloropropyl)morpholine CAS 7357-67-7 Gefi...
-
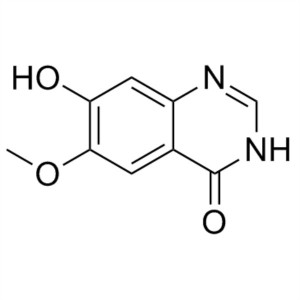
6-Methoxy-7-Hydroxyquinazolin-4-One CAS 162012-...